The Naming of Atoms
This is how it ought to be according to the official rules:
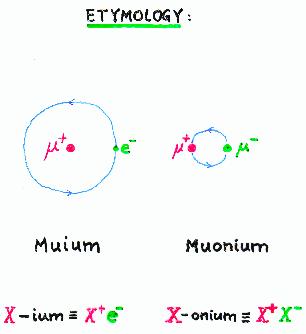
The official convention for naming atoms is that an
"X-onium" atom is composed of a particle and its
antiparticle, X+X-,
whereas an electronic atom formed with an X+
as its nucleus is called "X-ium".
Examples are "protonium" (p+p-)
vs. "protium" (p+e-)
and "positronium" (e+e-),
the last of which cleverly qualifies under both conventions.
But this isn't the way it is!
The µ+e- atom
(chemical symbol Mu) is not called "muium"
but instead is called "muonium", which properly ought
to refer to µ+µ-.
Why? The excuses are as follows:
- No one could pronounce "muium".
- The name became popular before the current conventions
were formally established, and you can't change all the literature
retroactively. (Otherwise electrons would be positively charged!)
- No one has ever observed
µ+µ-
and there are few prospects for doing so in the near future,
so there is no practical conflict.
Similar violations of convention apply for
"pionium" (Can you say "piium"?)
"kaonium" (How about "kaium"?)
and other electronic atoms with exotic nuclei.
Still another term, "exotic atom", is generally reserved for
atoms with normal nuclei in which an electron has been
replaced by a heavier negative particle.
The least exotic type is the muonic atom
µ-Z, in which the negative muon
often (for high atomic number Z) orbits so closely about the nucleus
that other electrons and neighbouring atoms "see" the
muonic atom as essentially a slightly enlarged and "fuzzy"
nucleus of charge +Z-1 and atomic weight A+0.113
(the muon mass is 0.01126 of the proton's).
But that's another story.
Author: JHB.
Figure created ~ 1985.
Prepared by
Jess H. Brewer
Last modified: Sat Nov 29 12:52:27 EST

